This website uses cookies to ensure a better user experience.
To get more information, please read our Cookie Statement.
Acceleration of hydrogen absorption by palladium through surface alloying with gold
Together with a group from University in Tokyo, Japan, our colleagues Petar Pervan, emeritus of the Institute Milorad Milun and alumni Ivo Pletikosić have published a paper in the prestigious journal Proceedings of the National Academy of Sciences about the acceleration effect of acceleration of hydrogen absorption by palladium through surface alloying with gold.
Acceleration of hydrogen absorption by palladium through surface alloying with gold
Kazuhiro Namba, Shohei Ogura, Satoshi Ohno, Wen Di, Koichi Kato, Markus Wilde, Ivo Pletikosić, Petar Pervan, Milorad Milun, and Katsuyuki Fukutani, PNAS July 13, 2018. 201800412
One of the important goals of „carbon-free“ technologies is the improvement of hydrogen cleansing membranes as well as hydrogen storage solutions. So far it is well known that Pd-Au alloys are able to absorb more hydrogen than pure palladium. However, the influence of gold on hydrogen solubility and its penetration into the bulk is not understood. This work looks into the absorption of hydrogen on surface alloys Pd-Au on top of Pd (110) using the techniques of therman desorption spectroscopy (TDS), hydrogen depth profiling with nuclear reaction analysis (NRA), angle-resolved photoemission spectroscopy (ARPES) as well as density functional theory.
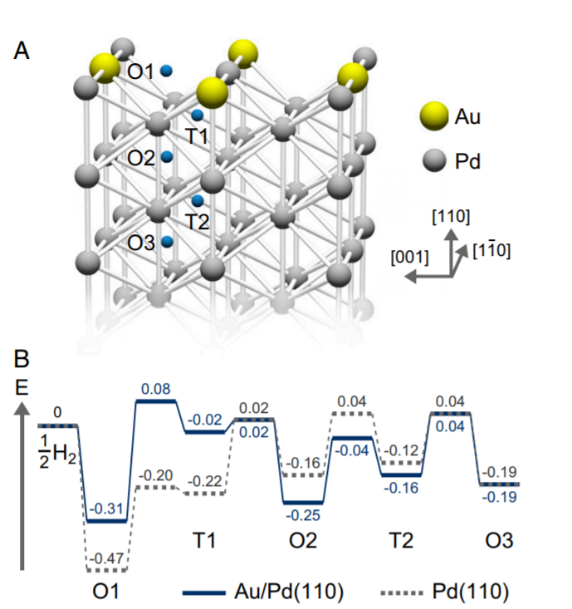
(A) Schematic model of 0,5-ML Au / Pd (110) used in DFT calculations. (B) Diagram of potential energy for an H atom on 0,5-ML Au/Pd (110) (solid line), on Pd (110) surface (dotted line)
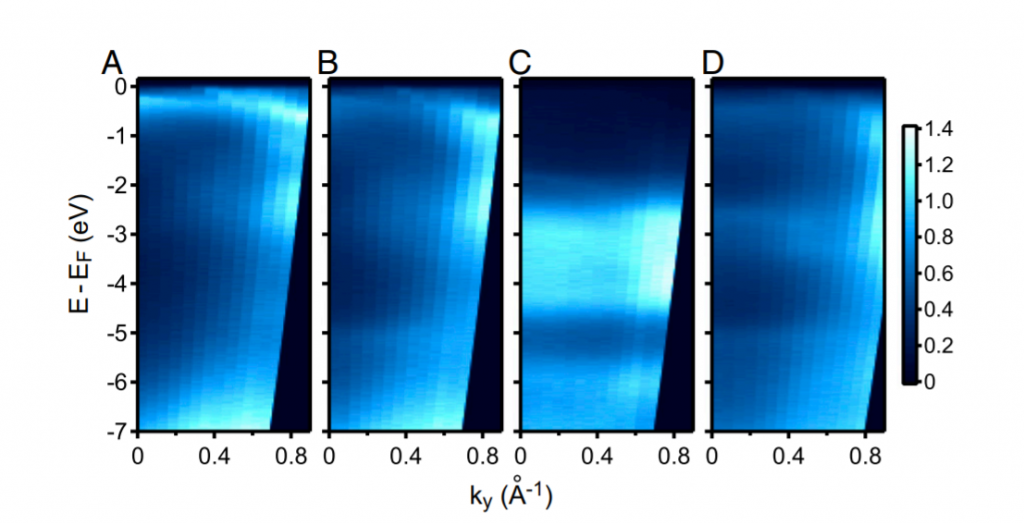
The papers shows that alloying the surface of Pd (110) with a sub-monolayer of Au dramatically accelerates hydrogen absorption.The degree of acceleration shows a volcano-shaped form against Au coverage. This enhancement of hydrogen kinetics can be explained by a reduced barrier of penetration which is caused by destabilizing the chemisorbed surface hydrogen, as supported by DFT calculations. The destabilization of chemisorbed surface hydrogen is ascribed to the change of surface electron states which has been observed by ARPES: it is well-known that the position of d-bands with respect to the Fermi level is a good indicator of the adsorption energy of surface molecules. The shift of d-bands toward higher binding energies is correlated with the reduction of adsorption energy of hydrogen which has clearly been seen by thermal desorption spectroscopy.
These findings can lead to a higher degree of control of hydrogen transport through Pd-based alloy surfaces as well as improve their selectivity and catalytic properties where adsorbed hydrogen in particular plays an important role.