This website uses cookies to ensure a better user experience.
To get more information, please read our Cookie Statement.
Ultrafast Photoelimination of Nitrogen from Upper Excited States of Diazoalkanes and the Fate of Carbenes Formed in the Reaction
Our ultrafast boys in collaboration with colleagues from Ruđer Bošković Institute published their work in Journal of Organic Chemistry where, using femtosecond laser spectroscopy, they investigated several photochemical reactions of carbene formation from diazoalkanes in anti-Kasha reactions from upper excited states.
Ultrafast Photoelimination of Nitrogen from Upper Excited States of Diazoalkanes and the Fate of Carbenes Formed in the Reaction
Vedran Brusar, Mateo Forjan, Ivan Ljubić, Marija Alešković, Kristin Becker,Silvije Vdović, Journal of Organic Chemistry 88, 7, 4286–4300 (2023).
DOI: 10.1021/acs.joc.2c02875
Carbenes are molecules with divalent carbon atoms that play key roles as organic reactive intermediates, as ligands in metal organic chemistry, and as highly potent organocatalysts. The chemistry of carbenes is controlled by their spin state, which is either triplet or singlet,and this spin dependent reactivity of carbenes forms the basis of carbene chemistry.
Photoreactions taking place from higher excited states, known as anti-Kasha photochemistry, are rare since not many photochemical processes can compete with fast internal conversion (IC) to the first excited electronic state (S1). Understanding ways to exploit the anti-Kasha reactivity has fundamental and technological value as it offers opportunities for a clever design of different photochemical processes where the selectivity of a reaction can be tuned by an appropriate choice of the excitation wavelength.
Photochemical reactivity of diphenyldiazomethane 1, and phenyl 1- and 2-adamantyl diazomethanes 2 and 3 was investigated experimentally by transient absorption spectroscopy (TA) with the time resolution from femtoseconds to milliseconds. Measurements provided spectroscopic evidence that the photoelimination of N2 upon excitation at 267 nm takes place in the anti-Kasha ultrafast photochemical reaction within 1 ps directly from the upper excited singlet states.
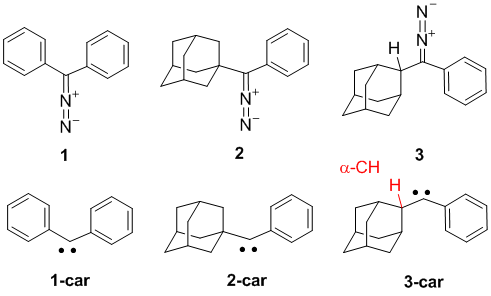
Figure 1. Investigated diazo derivatives 1-3 and the corresponding carbenes car-1 – car-3.
The N2 elimination delivers singlet carbenes, that were in the case of 1 and 2 detected by fs-TA. The reactivity of carbenes differs with respect to the substituent at the carbene center. The singlet car-1 in nonpolar solvent predominantly delivers the triplet carbene by intersystem crossing (ISC).
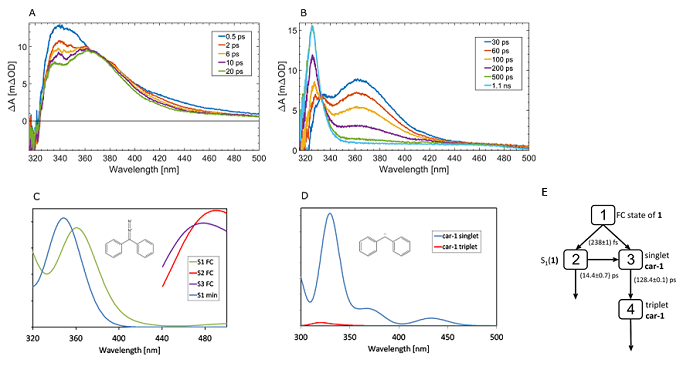
Figure 2. A) Transient absorption spectra of 1 upon excitation at 267 nm at early times. B) Later transient absorption spectra of 1 up to 1.1 ns probe delay. C) and D) The excited state absorption spectra computed for 1 and car-1. “S1 FC” and “S2 FC” are computed at the ground state (S0) minimum while “S1 min” is computed at the S1 minimum. The spectra are convoluted with the Gaussian profiles (fwhm = 3000 cm–1). E) Reaction model for target analysis of 1 that produced the best global fit results.
On the contrary, ISC for car-2 giving the corresponding triplet carbene was not detected. Instead, the singlet car-2 has longer lifetimes and reacts in the intermolecular insertion reactions into C-H bonds. Car-3 has an α C-H bond next to the carbene center, and therefore, reacts rapidly in the intramolecular C-H insertion reaction delivering alkene photoproduct, precluding its detection by fs-TA. However, isolation of ketone photoproducts from 3 was highly indicative of the formation of the triplet car-3 as a reactive intermediate. To confirm our initial assignment, the TA spectra from the S1, S2, and S3 excited states of 1, 2, and 3 were computed using time-dependent density functional theory (TD-CAM-B3LYP) while the multiconfigurational perturbation theory to the second order (CASPT2) was used for the absorption spectra of the corresponding singlet and triplet carbenes. The modeled and measured TA spectra are in good agreement, and the computations corroborate the assignments of the key short-lived intermediates in the photoinduced reactions of 1, 2, and 3.
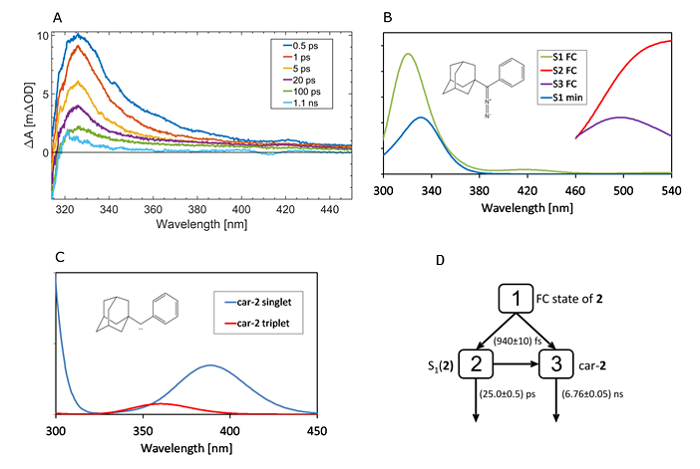
Figure 3. A) Transient absorption spectra of 2 upon excitation at 267 nm. B) and C) The excited state absorption spectra computed for 2 and car-2. “S1 FC” and “S2 FC” are computed at the ground state (S0) minimum while “S1 min” is computed at the S1 minimum. The spectra are convoluted with the Gaussian profiles (fwhm = 3000 cm–1). D) Reaction model for target analysis of 2 that produced the best global fit results.
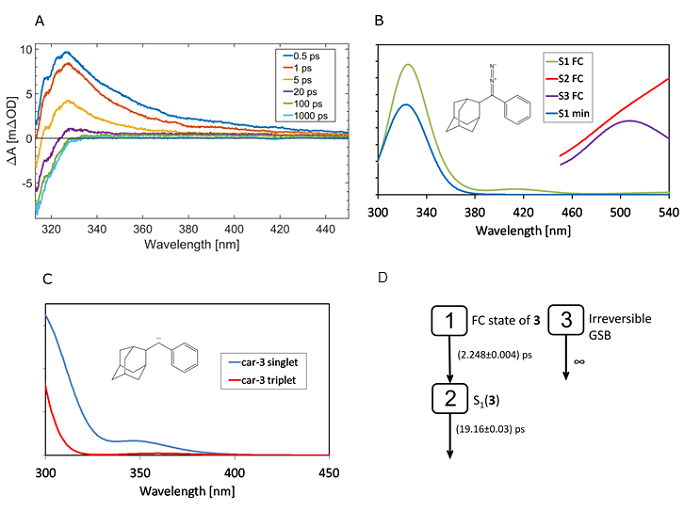
Figure 4. A) Transient absorption spectra of 3 upon excitation at 267 nm. B) and C) The excited state absorption spectra computed for 3 and car-3. “S1 FC” and “S2 FC” are computed at the ground state (S0) minimum while “S1 min” is computed at the S1 minimum. The spectra are convoluted with the Gaussian profiles (fwhm = 3000 cm–1). D) Reaction model for target analysis of 3 that produced the best global fit results.